The immune system is a highly coordinated biological defense network designed to protect the body from infections, malignancies, and other foreign threats. Among its most important cellular components are T lymphocytes (T cells), which orchestrate and execute immune responses. One of the most frequently asked questions in immunology is: Are CD4 T cells helper or cytotoxic?
At first glance, this seems straightforward. CD4 T cells are classically known as helper, while CD8 T cells are recognized as cytotoxic. However, modern immunological research has revealed a much more nuanced reality. CD4 T lymphocytes are primarily helper cells, but under specific pathological or physiological conditions, they can also acquire cytotoxic functions.
By the end of this blog, you will have a clear, evidence-based understanding of CD4 T cell biology, their helper roles, cytotoxic potential, and clinical significance.
What Are CD4 T Cells?
CD4 T cells are a subset of T lymphocytes that express the CD4 glycoprotein on their surface. This molecule functions as a co-receptor that enhances the interaction between the T cell receptor (TCR) and major histocompatibility complex class II (MHC II) molecules.
Key Characteristics of CD4 T Cells
- Develop in the thymus through positive and negative selection
- Recognize antigens presented by MHC class II molecules
- Primarily interact with professional antigen-presenting cells (APCs) such as:
- Dendritic
- Macrophages
- B lymphocytes
Once activated, CD4 T lymphocytes differentiate into functionally specialized subsets that shape immune responses through cytokine secretion and cell-to-cell interactions.
Why Are CD4 T Cells Predominantly Called Helper T Cells?
CD4 T cells are termed helper T cells (Th) because their primary role is to assist and regulate the activity of other immune cells rather than directly killing infected ones. Once activated, CD4 T lymphocytes secrete a wide range of cytokines, which are soluble signaling proteins that influence immune cell growth, differentiation, and activation.
Helper functions of CD4 helper T cells include:
- Stimulate B cells to produce antibodies
- Enhance macrophage phagocytosis and microbial killing
- Support the differentiation and survival of CD8 cytotoxic T cells
- Regulate immune homeostasis and tolerance
When CD4 T lymphocytes are depleted, patients become highly susceptible to opportunistic infections. Thus, the term “helper” reflects the essential role these cells play in immune defense.
Subsets of CD4 Helper T Cells
Following activation, naïve CD4 T cells differentiate into specialized subsets depending on cytokine signals in their environment. Each phenotype secretes a different profile of cytokines, tailoring responses to specific pathogens and immune threats.
Th1:
They drive cell-mediated immunity by secreting interferon-γ (IFN-γ) and tumor necrosis factor (TNF), which activate macrophages and enhance phagocytic killing. Also, they are crucial for defense against intracellular pathogens such as viruses and intracellular bacteria.
Th2:
They regulate humoral immunity through the production of interleukin-4, interleukin-5, and interleukin-13, supporting B-cell antibody production, class switching, and eosinophil activation. While these protect against extracellular parasites, dysregulated Th2 responses can contribute to allergic and atopic diseases.
Th9:
They are mainly activated in allergic inflammation and immune defense against helminthic infections. Their effects are mediated by IL-9, which strengthens type 2 immune responses at mucosal and epithelial surfaces.
Th17:
They are pro-inflammatory CD4 T subsets concentrated at mucosal barriers, where they support barrier immunity. By producing IL-17, IL-21, IL-22, IL-25, and IL-26, they recruit neutrophils to protect against extracellular bacteria and fungi. Th17 are also linked to autoimmune pathology.
Th22:
They are mostly found in the human skin and primarily secrete IL-22 to support tissue repair. Moreover, they promote keratinocyte proliferation while maintaining epidermal integrity during inflammation and wound healing.
Regulatory T Cells (Tregs):
They maintain peripheral tolerance by controlling excessive immune activation. This subset also expresses the FOXP3 transcription factor and suppresses immune responses mainly through IL-10 and TGF-β, thereby preventing autoimmune disease.
T Follicular Helper (Tfh) Cells:
They support B-cell responses to produce antibodies against pathogens within germinal centers. By secreting cytokines IL-21, they drive B-cell proliferation, affinity maturation, and long-term humoral immunity. All these subsets reinforce the helper identity of CD4 T lymphocytes.
Are CD4 T Cells Cytotoxic? The Scientific Explanation
Unlike conventional helper CD4 T cells that primarily coordinate immune responses through cytokine secretion and cellular crosstalk, cytotoxic variants are capable of directly killing infected, transformed, or malignant cells. Importantly, they recognize antigenic peptides presented on MHC class II molecules, allowing them to directly target infected or malignant cells that are often invisible to classical CD8 cytotoxic T lymphocytes.
Although they are primarily helpers, extensive research shows that a subset can acquire cytotoxic properties under certain conditions. These have been identified in chronic viral infections, cancer, and inflammatory diseases.
Cytotoxic CD4 T Cells Can:
- Express perforin and granzymes (particularly granzyme B), enabling granule-mediated apoptosis of target cells
- Directly kill infected or malignant cells that express MHC class II molecules
- Induce programmed cell death (apoptosis) via Fas–FasL interactions
- Complement CD8 T cell responses by recognizing distinct antigenic epitopes
These cytotoxic CD4 T cells are sometimes referred to as CD4 CTLs (cytotoxic T lymphocytes).
When Do CD4 T Cells Become Cytotoxic?
Cytotoxic CD4 T cells are not the default state but arise under prolonged antigen exposure or inflammatory signaling. Experimental and clinical studies have identified cytotoxic CD4 T lymphocytes in these conditions:
- Chronic viral infections (e.g., persistent antigen exposure, such as CMV, HIV, hepatitis viruses, EBV, and influenza)
- Tumor microenvironments
- Certain autoimmune and inflammatory diseases
- Aging and immune system remodeling
Their existence highlights the remarkable adaptability of the immune system and challenges the traditional rigid classification of T cell functions.
Mechanisms of CD4 Cytotoxicity
Cytotoxic CD4 T cells use molecular mechanisms similar to those used by CD8 T lymphocytes. Their cytotoxic function is mediated through two principal pathways:
Perforin–Granzyme Pathway
Following antigen recognition, activated CD4 cytotoxic T cells release perforin, which forms pores in the target cell membrane. Granzymes then enter the cell through these transient pores, triggering caspase activation and programmed cell death.
Fas–Fas Ligand Pathway
Cytotoxic CD4 T cells can also express Fas ligand (FasL), which binds to Fas (CD95) receptors on target cells. This interaction initiates an intracellular apoptotic cascade, leading to controlled cell death. Beyond direct killing, cytotoxic CD4 T lymphocytes often secrete inflammatory cytokines such as interferon-γ and tumor necrosis factor-α, which enhance antigen presentation and strengthen local immune responses.
While effective, these mechanisms are typically less efficient than those of CD8 T cells. Cytotoxic CD4 T cells, therefore, act as supplementary effectors, especially when CD8 T responses are impaired or insufficient.
CD4 Helper T Cells and CD8 Cytotoxic T Cells
CD4 helper T cells and CD8 cytotoxic T cells represent two major functional lineages of adaptive T lymphocytes. Although both subsets arise from naïve T cells and contribute to immunological memory, their roles within immune responses are distinct.
The following table summarizes the key phenotypic and functional differences between the two.
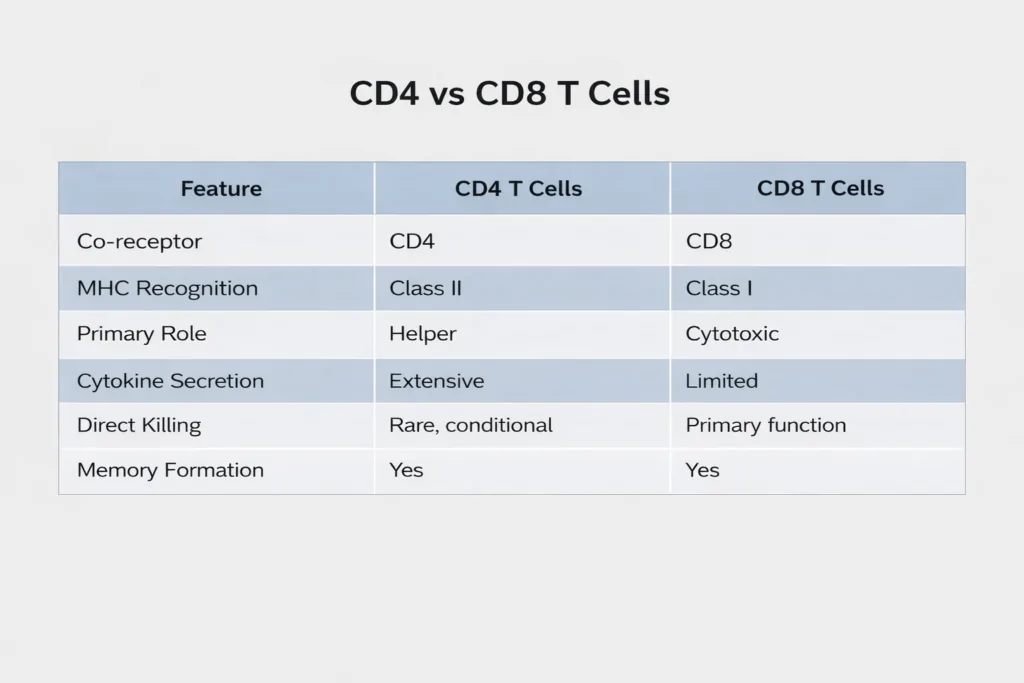
This comparison clearly shows that cytotoxicity is central to a CD8 T cell, while a CD4 T cell is primarily regulatory and supportive.
Memory CD4+ T Cells and Long-Term Immune Protection
Memory CD4 T lymphocytes are essential for rapid and efficient immune responses during secondary exposure to pathogens. Once an initial pathogen is cleared, a fraction of activated CD4 T cells differentiates into memory cells that persist for extended periods. These remain in a poised state, allowing them to respond rapidly and effectively when the same antigen is encountered again.
Compared with naïve CD4 T cells, memory CD4 T cells exhibit several functional advantages:
- Produce effector cytokines more rapidly upon antigen re-exposure
- Provide enhanced and faster help to B cells for antibody production
- Support robust activation and maintenance of CD8+ cytotoxic T cells
- In certain immune settings, they can also retain cytotoxic capabilities
This clearly demonstrates that CD4 T cells cannot be defined by a single role. Because of their functional versatility, memory CD4 T cells provide both immune coordination and direct protection.
Clinical and Medical Significance of CD4 T Cells
CD4 T cells are central to human health and disease. Their depletion or dysfunction has profound clinical consequences across diseases, such as:
1. Infectious Diseases
In HIV infection, the virus selectively targets CD4 T cells, leading to progressive immune failure and susceptibility to opportunistic infections. Hence, CD4 T cell depletion is a hallmark of HIV infection.
2. Antiviral Immunity
By releasing cytokines such as IFN-γ and TNF-α, CD4 T cells help control viral replication while coordinating both innate and adaptive immune responses. Virus-specific CD4 T cells from recovered individuals, including those from COVID-19, have shown promise as a source of long-lasting cell-mediated antiviral protection.
3. Cancer Immunology
CD4 T cells enhance antitumor immunity by supporting cytotoxic T lymphocytes, activating macrophages, and, in some cases, directly killing tumor cells. Their ability to regulate angiogenesis and tumor dormancy makes them critical targets in cancer immunotherapy.
4. Autoimmune Disorders
Dysregulated CD4 T cell activation contributes to autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease. Therapeutic strategies often aim to rebalance specific helper T cell subsets to restore immune tolerance rather than broad immunosuppression.
5. Biomarkers in Immune and Neurological Disorders
CD4 T lymphocytes serve as important cellular markers to evaluate immune competence and diagnose primary immunodeficiencies through flow cytometry. Additionally, CD4 T cell–derived microRNAs have emerged as promising biomarkers for neurodegenerative conditions such as Alzheimer’s disease.
6. Transplantation and Graft Rejection
Memory CD4 T cells play a significant role in antibody-mediated graft rejection and long-term transplant failure. Understanding their sensitization and effector mechanisms has advanced the development of experimental models aimed at improving graft tolerance and transplant success.
Final Answer: Are CD4 T Cells Helper or Cytotoxic?
The definitive answer is:
- CD4 T cells are primarily helper T cells
- They regulate, coordinate, and amplify immune responses by providing essential cytokine signals that guide the activity of B cells, macrophages, and CD8 cytotoxic T cells
- A specialized subset can exhibit cytotoxic activity, particularly under conditions of chronic infection, inflammation, or tissue-specific immune responses, but this is not its dominant function
Thus, CD4 T lymphocytes should be understood as functionally flexible immune regulators, with helper activity at their core and cytotoxic potential under specific biological conditions.
Conclusion
CD4 T cell represents one of the most versatile and essential components of the adaptive immune system. While historically classified as helper T lymphocytes, modern immunology has expanded this view to include their potential cytotoxic capabilities. Nevertheless, their defining role remains immune coordination rather than direct killing. This dual functionality reflects the remarkable complexity of immune regulation and explains why CD4 T lymphocytes are central to protective immunity, disease pathogenesis, and the advancement of immune-based therapies.
Frequently Asked Questions (FAQs)
What are CD4 T cells?
CD4 T cells are a subset of T lymphocytes that express the CD4 co-receptor and primarily regulate immune responses by recognizing antigens presented on MHC class II molecules.
What’s the difference between CD4 and CD8 T cells?
The purpose of CD4 T cells is to coordinate immune responses through cytokine secretion, whereas CD8 T cells essentially kill infected or malignant cells directly.
What is the importance of a CD4 T cell?
CD4 T cells are essential because they activate and regulate B cells, macrophages, and CD8 T cells, making them central to effective immune defense.
Are CD4 T lymphocytes cytotoxic or helper?
CD4 T cells are predominantly helper cells, although a specialized subset can exhibit cytotoxic activity under certain conditions.
Can CD4 be cytotoxic?
Yes, CD4 T lymphocytes can become cytotoxic in settings such as chronic infections, cancer, or prolonged inflammation.
What are the cytotoxic T cells?
Cytotoxic T cells are mainly CD8 T lymphocytes that kill infected or cancerous cells using perforin–granzyme and Fas–FasL–mediated mechanisms.
What is the full name of CD4 T?
Cluster of Differentiation 4–positive T lymphocytes.
Want to Learn More?
To learn more about this topic, check out our recent articles on T cell biology and CD4+ T Lymphocytes.