Since 1891, scientists have been working to develop immunotherapy treatments for cancer patients. Not surprisingly, researchers have made important advancements over the past 130 years in using a patient’s immune system to fight cancerous cells more effectively.
We are now at an inflection point in the history of immunotherapy, as adoptive cell transfer (ACT) approaches have demonstrated breakthrough potential in treating cancer and other diseases. ACT involves the transfer of cells to a patient, and the most successful variants have been CAR T-cell therapies.
To learn more about these revolutionary advancements in cancer treatment, keep reading. We’re going to break down these therapies into their basic concepts and highlight how clinicians now have expanded treatment options for patients because of them.
Understanding Different Cell Therapies
Before exploring how researchers use CAR T and T cells in cancer therapy, you need to understand how T cells and CAR T-cell therapies works. Even if you have a basic understanding of the interactions, it’s important to refresh your memory before diving into the research.
How T Cells Work
T cells are a type of white blood cell that play a critical role in the human immune system. These cells orchestrate the body’s immune response. In addition, they kill cells that have become infected by pathogens.
Simply put, T cells are the key to the human immune system and adaptive immunity. Thus, scientists have opted to use these cells to develop new therapies for patients battling cancer.
Researchers have used T cells in basic and preclinical research for many years. But, CAR T-cell therapy has introduced a new frontier in immunological research.
How CAR T-Cell Therapies Work
Cancer cells have the ability to avoid detection by the immune system. For example, cancer cells can disguise themselves as healthy cells or sprout so many antigens on their surface that T cells are stymied and can’t mount an effective attack. There are also a number of other ways that cancer can turn off the immune system against them.
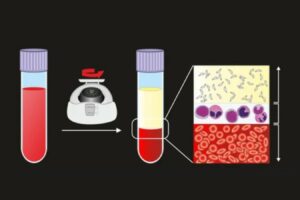
One innovation to see through the deception of cancer cells is CAR T-cell therapy. The process for delivering a CAR T-therapy starts with collecting a patient’s own T cells similar to a blood donation.
Next, a modified and inactive virus is transduced into the T cells to introduce genetic information. This process reprograms the T cells so they produce special receptors called chimeric antigen receptors, or CARs, on their surface.
These newly engineered cells, called CAR T-cells, are grown in a bioreactor and millions of them are infused back in the patient. The new receptors enable the T cells to latch onto a specific antigen on the patient’s tumor cells and destroy them.
CAR T-Cell Manufacturing Process

Modern CAR T-Cell Therapy
Until recent developments, researchers were only permitted to use CAR T-cell therapies in clinical trials with a small number of participants. The patients in those trials were typically far advanced in their disease.
However, recent clinical successes has led regulatory approvals and proliferation of this therapeutic concept to treat different types of cancer.
In fact, the Food and Drug Administration (FDA) has approved four CAR T-cell therapies so far. One of the therapies, KymriahTM is for patients up to 25 years old with acute lymphoblastic leukemia (ALL). The other therapies are for adults with advanced lymphomas.
Even with all of this hope, there are still many unknowns. Many researchers still consider CAR T-cell therapies to be a nascent field of biomedical research.
Fortunately, biopharma companies and academic researchers across the world are conducting studies on CAR T-cells to expand their use for all forms of cancer, not just blood cancers. This research is also leading to increased safety and effectiveness of existing therapies.
Recorded Side Effects in Studies With CAR-T Cells
Cancer therapies are known for their many side effects. Unfortunately, the side effects of CAR T-cell therapies can be challenging to manage because of the limited evidence base and experience in this emerging field.
One of the most frequent side effects that has occurred in patients who’ve received CAR T-cell therapy is cytokine release syndrome, or CRS.
CRS occurs when CAR T-cells start to expand in patients with hematological malignancies, usually within their bone marrow. When the T cells start to expand and attack the cancer they release cytokines which can cause an inflammatory response.
In patients with CRS, T cells release too many cytokines and can lead to high fever, low blood pressure, and organ toxicity problems affecting renal function and liver function. These side effects have the potential to be fatal and must be closely monitored during treatment.
The Future of CAR T-Cell Therapy
Despite the impressive initial response rates in blood cancer patients who have been treated with the currently approved CAR T-cell therapies, about half these patients relapsed within a couple years.
The primary reason for this is that CAR T-cells can become “exhausted,” which allows any residual cancer cells to proliferate and lead to a full-blown relapse. To address this, scientists have explored highly engineered “exhaustion-proof” CAR T-cells and combining CAR T-cells with other immunotherapy approaches.
The field of CAR T therapy is one of the most creative and innovative fields in oncology. This is because the genetic engineering opens up the possibilities to treat a wide range of diseases more effectively.
By removing a gene or introducing a synthetic receptor like a CAR, scientists can create cells that have properties that go beyond what natural cells can do. This may lead to more targeted therapies and personalized medicine.
It’s still too early to understand the full potential of CAR T-cells, but clearly the challenge of identifying suitable targets and further refining the CAR design will be the focus for researchers for years to come.
Developing a CAR T-cell therapy for solid tumor cancers is a “holy grail” pursuit for many scientists. The challenges here are quite formidable. But seeing how far the field has come in the last decade, there’s reason to believe we will overcome these challenges and expand access of immunotherapy to more patients.
Source Material for CAR T-Cell Therapy Research
As research in the field of CAR T-cell therapies continues to grow, developers need access to quality immune cell products to evaluate the potential of new therapeutic concepts.
At Cytologics, we’re working to improve the effectiveness of CAR T-cell therapy and expand the types of cancers for which it can be used.
We are proud to supply pharmaceutical, biotech and academic customers with quality T cells and other lymphocytes to support their cell therapy research.
To learn more about our products and services, contact us or submit a custom request today!